News
ALL NEWSResearch
July 09, 2023
New insights into liver cancer development:
Identified a novel mechanism by which liver cancer develops, involving a gene called GREB1 and aberrant activation of a signaling pathway known as Wnt
(Prof.A.Kikuchi, in Cancer Research)
Overview of the Research
Specially Appointed Professor Akira Kikuchi, Center for Infectious Diseases Education and Research, Osaka University, along with Associate Professor Shinji Matsumoto and Assistant Professor Akikazu Harada, Graduate School of Medicine, Osaka University, have identified a novel cancer signaling axis and therapeutic target for hepatocellular carcinoma (HCC, liver cancer) treatment. Aberrant activation of the Wnt signaling pathway maintains two cellular states, differentiation and proliferation, in liver cancer. On the other hand, activation of Wnt signaling in colorectal cancer stimulates proliferation while keeping cells undifferentiated. To elucidate the differences in the mechanism of action of Wnt signaling in liver and colorectal cancer, comprehensive analysis of liver cancer-specific Wnt signaling target genes using TCGA database identified GREB1 as a novel target gene. When Wnt signaling is abnormally activated in liver cancer, transcription factor TCF4 induced GREB1 expression in cooperation with HNF4α and FOXA2, which are master transcription factors that maintain the differentiation of hepatocytes. Furthermore, induced GREB1 bound to the regulatory region of proliferation-promoting genes along with HNF4α, promoting the proliferation of liver cancer cells. Therefore, it was revealed that GREB1 stimulates proliferation under the differentiation state in liver cancer by utilizing differentiation factor HNF4α.
In Japan, approximately 25,000 people die from liver cancer every year. Recently, the development of immunotherapy checkpoint inhibitors and multi-kinase inhibitors as drug therapies for liver cancer has improved prognosis, but there are cases that do not respond to these treatments, and there is a need for the development of new molecular targeted therapies. In particular, liver cancer with activated Wnt signaling due to β-catenin mutation becomes immune cold, failing to respond to immunotherapy checkpoint inhibitors. Knockdown of GREB1 decreased tumor proliferation of liver cancer cells, suggesting that GREB1 may be a novel therapeutic target for liver cancer.
This study was published in Cancer Research on 22nd June, 2023.
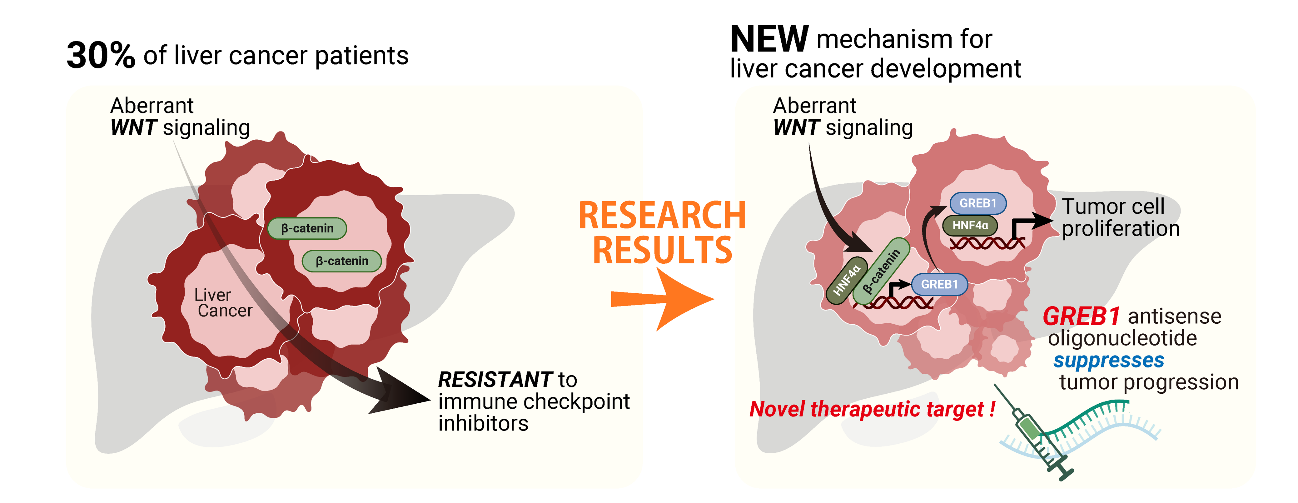
Fig.
Schematic representation of this study
Credit: Akikazu Harada
Background of the Study
The Wnt signaling pathway is involved in embryonic development and stem cell regulation, and is also closely related to cancer development. Tumor formation through the aberrant activation of Wnt signaling is frequently observed in malignant tumors such as colon cancer, where it promotes proliferation of undifferentiated stem cells. Liver cancer is also one of the tumors with a high frequency of activation of Wnt signaling. In hepatocytes located near the central vein in the normal liver, Wnt signaling is physiologically activated, and those differentiated hepatocytes serve as the origin of liver cancer development. Unlike in intestinal stem cells, proper activation of Wnt signaling in hepatocytes physiologically regulates the expression of metabolic genes such as glutamine synthetase (GS) and cytochrome P450 enzymes (CYPs), maintaining hepatocyte differentiation. However, in liver cancer development, the aberrant activation of Wnt signaling stimulates cell proliferation while maintaining hepatocyte differentiation. Therefore, in liver cancer, aberrant activation of Wnt signaling maintains the contradictory states of differentiation and proliferation, but the molecular mechanisms underlying this phenomenon have not been fully elucidated.
GREB1 (Growth regulation by estrogen in breast cancer 1) is a gene of which expression is induced by estrogen in breast cancer cells. Estrogen receptor alpha (ERα), a nuclear receptor, binds to the promoter region of GREB1 and induces its expression, and the induced GREB1 enhances the transcriptional activity of ERα by binding to it. GREB1 is also induced by androgen in androgen receptor (AR) positive prostate cancer cells. Furthermore, knockdown of GREB1 has been shown to inhibit the proliferation of breast cancer and prostate cancer cells, suggesting that GREB1 stimulates proliferation of sex hormone-dependent cells. In this study, Professor Kikuchi’s group revealed that GREB1 is a liver cancer-specific Wnt signaling target gene and acts as a novel mediator of the Wnt signaling pathway, regulating unique mechanism of proliferation under differentiated cell state in liver cancer development.
Research Achievements
1.A novel algorithm was developed, and it was applied to expression profile of TCGA in order to explore liver cancer specific Wnt signaling target genes. Among 46 candidates identified, GREB1 was the top-ranked gene which was associated with cell proliferation and whose expression was suppressed by knockdown of β-catenin in liver cancer cells. Therefore, we defined GREB1 as a novel liver cancer-specific Wnt signaling pathway target gene.
2.In a collaborative study with Professor Takumi Fukumoto and Assistant Professor Hidetoshi Gon, Department of Hepato-Biliary-Pancreatic Surgery, Graduate School of Medicine, Kobe University, the research group created a liver cancer tissue microarray consisting of 427 cases and performed immunohistochemical analysis of the expression of β-catenin and GREB1, comparing them with clinicopathological findings. As a result, the expression of GREB1 and β-catenin was observed in 32% and 37% of the cases, respectively, and there was a positive correlation between their expressions. On the other hand, GREB1 expression did not affect the 5-year survival rate and was observed in cases with well-differentiated liver cancer. These results are consistent with the finding that β-catenin expression is frequently observed in well-differentiated liver cancer.
3.From the TCGA database, the research group extracted 200 genes that show a positive correlation with GREB1 expression in liver cancer and performed enrichment analysis, which revealed that HNF4α and FOXA2 transcriptionally regulates expression of these genes. They also performed ChIP sequencing in liver cancer cells, and HNF4α, FOXA2, TCF4, β-catenin, and acetylated H3K27 were found to accumulate in the upstream 34 kb region of the transcription start site of GREB1, and this region (En) was considered to be a super-enhancer. Furthermore, when this region was knocked out in liver cancer cells, GREB1 expression indeed decreased, and tumor-forming ability also decreased.
4.GREB1 was not expressed in colorectal cancer although Wnt signaling was highly activated. In the En region of GREB1 in colorectal cancer cells, accumulation of HNF4α, FOXA2, and acetylated H3K27 was not observed, but instead, methylated H3K27, a chromatin inactivation marker, was accumulated.
5.RNA sequencing analysis was performed after knocking down GREB1 in liver cancer cells, and revealed that down-regulated genes were related to cell proliferation. Furthermore, GREB1 knocked out liver cancer cells showed decreased in vitro and in vivo cell proliferation ability.
6.Through ChIP sequencing of GREB1, GREB1 was found to be colocalized at the genomic regions together with HNF4α and FOXA2. The complex between GREB1 and HNF4α, and GREB1 and FOXA2 was formed in liver cancer cells, and the interaction of HNF4α with FOXA2 was inhibited by GREB1 knockdown.
7.Ninety-five genes that are upregulated in a GREB1- and HNF4α-dependent manner were identified, and they were named TR95 (target 95). TR95 includes driver genes of liver cancer such as IGF2 and LIN28B, and its expression was decreased when GREB1 was knocked down. The expression of TR95 in TCGA database was also correlated with the expression of GREB1 and HNF4α, and associated with poor prognosis of liver cancer.
8.For the purpose of developing a novel treatment for liver cancer, GREB1 antisense oligonucleotide (ASO) was designed and administered to a mouse liver cancer model, resulting in a decrease in the expression of GREB1 and TR95, and the inhibition of tumor formation.
Significance of the Research Findings
This research has revealed the molecular mechanism by which the aberrant activation of Wnt signaling promotes cancer cell proliferation with maintaining the differentiation state in lover cancer. When Wnt signaling is activated in the basal level in normal hepatocytes, GREB1 is only minimally expressed. However, when Wnt signaling is aberrantly activated due to mutations in β-catenin in differentiated hepatocytes, β-catenin/TCF4 and master transcription factors for liver cell differentiation, HNF4α and FOXA2, binds to the upstream 34 kb region (En) of the GREB1 gene, promoting GREB1 expression. Furthermore, GREB1 collaborates with HNF4α to promote the expression of a set of genes (TR95) involved in cell proliferation. In other words, HNF4α, which has been considered as a transcription factor for hepatocyte differentiation and a tumor suppressor, has been shown to promote liver cancer cell proliferation with activated Wnt signaling. Thus, GREB1 induces an oncogenic shift of HNF4α. Additionally, the anti-tumor effects of GREB1 antisense oligonucleotide (ASO) have been demonstrated, suggesting that GREB1 may be a novel therapeutic target for liver cancer. As liver cancer with activated Wnt signaling are resistant to immune checkpoint inhibitors, the development of new treatment strategies is desired. It is expected that this research finding will contribute to the novel drug development.
Special Notes
This research finding was published in Cancer Research on 22nd June, 2023.
Title: Wnt signaling stimulates cooperation between GREB1 and HNF4α to promote proliferation in hepatocellular carcinoma
Authors: Shinji Matsumoto†*, Akikazu Harada†(†equally contributed), Minami Seta, Masayuki Akita, Hidetoshi Gon, Takumi Fukumoto, Akira Kikuchi*(*corresponding authors)
DOI:https://doi.org/10.1158/0008-5472.CAN-22-3518
This research was supported by Grants-in-Aid for Scientific Research (S) and the AMED-Project for Promotion of Cancer Research and Therapeutic Evolution.
ResOU: https://resou.osaka-u.ac.jp/en/research/2023/20230704_1