News
ALL NEWSResearch
March 27, 2023
Clinical significance of the activation of DKK1-CKAP4 signaling axis in hepatocellular carcinoma and application for the development of new cancer therapy
(Prof.A.Kikuchi,et al. in Cancer Sci.)
Overview of the Research:
Professor Akira Kikuchi (Center for Infectious Disease Education and Research, Osaka University), along with Associate Professor Shinji Matsumoto and Assistant Professor Ryota Sada, and post-graduate student Kosuke Iguchi (Graduate School of Medicine, Osaka University), revealed that DKK1-CKAP4 signaling is activated in hepatocellular carcinoma (liver cancer), and that CKAP4 becomes a new molecular target for liver cancer therapy. In Japan, about 25,000 people die due to liver cancer every year. Although immune checkpoint inhibitors and multi-kinase inhibitors have been developed, there are some cases that are unresponsive to these drugs, hence the development of new molecular targeted therapies is required.
In this study, Professor Kikuchi's group found that the novel cancer signal, the DKK1-CKAP4 pathway, is activated in liver cancer and that its activation promotes liver cancer growth. In addition, they showed that the anti-CKAP4 antibody inhibits the proliferation
of liver cancer cells using in vivo mouse model. Furthermore, they discovered that the anti-tumor effect is enhanced by combining anti-CKAP4 antibody with the standard treatment drug, lenvatinib, which could lead to the development of a new therapeutic strategy. These research achievements were published in Cancer Science.
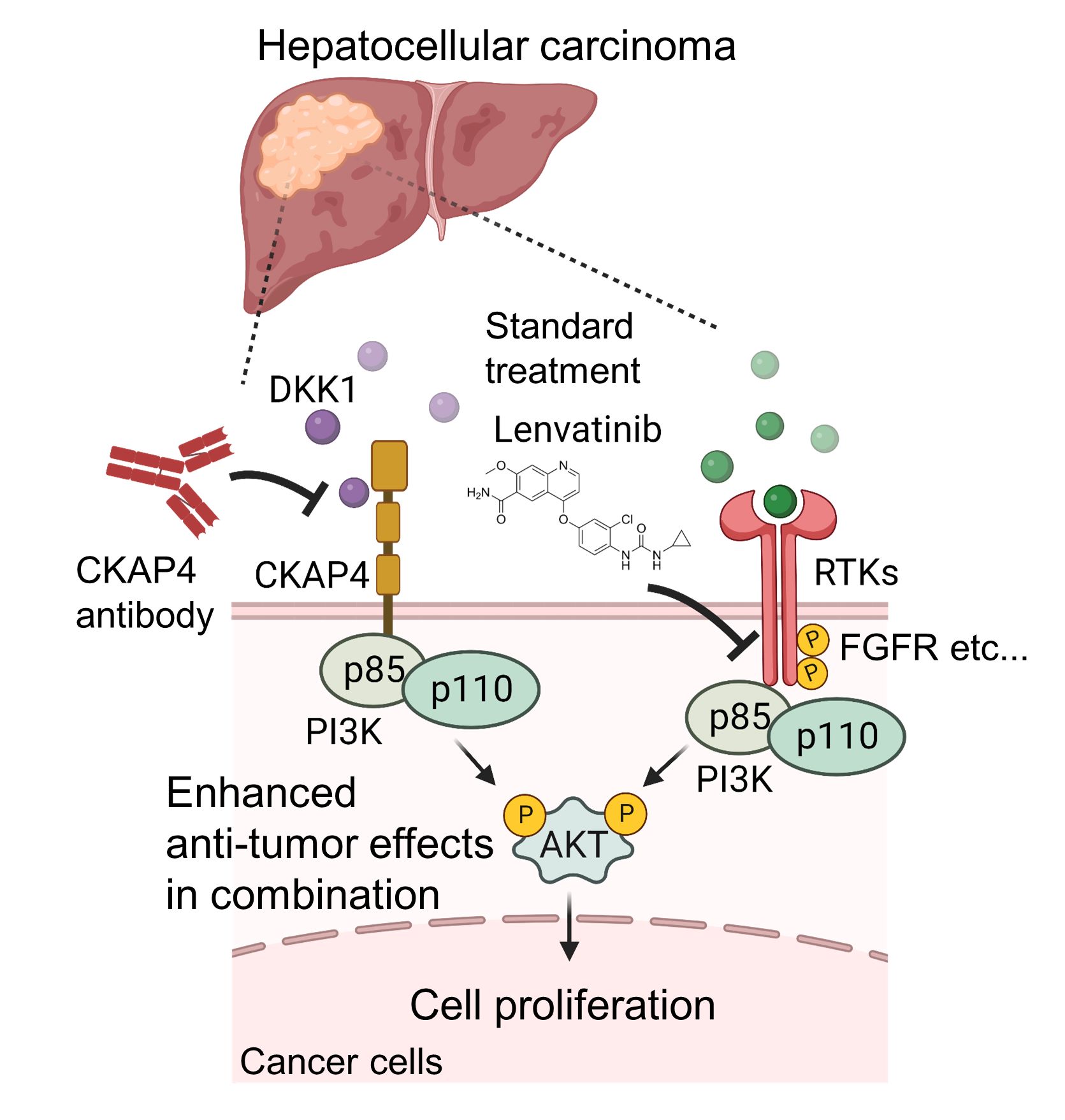
Fig.
Usage restriction: Cannot be used without permission
Credit: Kikuchi et al.
Research Background
Dickkopf1 (DKK1) is an extracellular secreted protein, which had been known to promote the growth of various cancers, but its molecular mechanisms were unknown for many years. Professor Kikuchi's group identified Cytoskeleton-Associated Protein 4 (CKAP4) as a receptor for DKK1 in 2016. When DKK1 binds to CKAP4, the PI3 kinase-AKT pathway is activated, and cancer cell growth is promoted. It was also found that the anti-CKAP4 antibody blocks DKK1-CKAP4 signaling and inhibits cancer cell proliferation. Kikuchi’s group has also reported that the DKK1-CKAP4 pathway is activated in pancreatic cancer, lung cancer, and esophageal cancer, and is correlated with poor prognosis of these cancers. Furthermore, they have demonstrated that the anti-CKAP4 antibody has anti-tumor effects in mouse models of these refractory cancers. In this study, they used 412 cases of liver cancer to determine whether the DKK1-CKAP4 pathway is activated and investigated whether the anti-CKAP4 antibody shows anti-tumor effects against liver cancer cells.
Research Results
1.This study aimed to investigate the activation of the DKK1-CKAP4 signaling pathway in liver cancer and the potential of the anti-CKAP4 antibody to show antitumor effects against liver cancer cells in collaboration with Professor Takumi Fukumoto and Assistant Professor Hidetoshi Gon (Graduate School of Medicine, Kobe University). Using a liver cancer tissue microarray consisting of 412 cases, they analyzed the expression of DKK1 and CKAP4 by immunohistochemistry and compared the expression of these proteins with clinical and pathological findings. The data found that cases expressing both DKK1 and CKAP4 exhibited aggressiveness and poor prognosis than cases expressing either one or neither (CKAP4 was confirmed to be indeed present in the plasma membrane to function as a receptor).
2.They also confirmed the expression of DKK1 and CKAP4 in nine liver cancer cell lines and found that Hep3B, JHH7, and HuH-7 cells highly expressed both proteins. In vitro and in vivo experiments showed that knockdown of the expression of DKK1 or CKAP4 in Hep3B and JHH7 cells decreases AKT activity and reduces their proliferation ability.
3.Furthermore, they investigated the combination effect of anti-CKAP4 antibody and lenvatinib, a multi-kinase inhibitor commonly used for liver cancer treatment and found that their combination showed a synergistic effect in inhibiting AKT activity and Hep3B cell growth in a mouse model.
Significance of Research Findings
From the results of this study, both DKK1 and CKAP4 were found to be highly expressed in 55 out of 412 liver cancer cases (13.3%), which were more malignant and had poor prognosis. This means that DKK1-CKAP4 signaling is more activated by the expression of a ligand, DKK1, and a receptor, CKAP4, leading to the promotion of cancer cell proliferation. These results were consistent with those of previous studies on pancreatic, lung, and esophageal cancer cases. Furthermore, as the anti-CKAP4 antibody inhibited liver cancer cell proliferation, CKAP4 is considered to be a novel molecule target for liver cancer treatment, and a new treatment strategy is suggested by combining anti-CKAP4 antibody with lenvatinib to enhance the anti-tumor effect additively. There are some liver cancer cases that are unresponsive to standard treatment, so the development of new treatments is desired. It is expected that the present research will contribute to this.
Overall, this study suggests
that DKK1-CKAP4 signaling is activated in liver cancer and that targeting CKAP4 with the anti-CKAP4 antibody could be a potential therapeutic strategy for liver cancer, especially when used in combination with other standard treatments.
The article, “The DKK1-CKAP4 signal axis promotes hepatocellular carcinoma aggressiveness” was published in Cancer Science on January 31, 2023.
【Title】
The DKK1-CKAP4 signal axis promotes hepatocellular carcinoma aggressiveness
【Journal】
Cancer Science
【Authors】
Kosuke Iguchi, Ryota Sada, Shinji Matsumoto, Hirokazu Kimura, Yoh Zen, Masayuki Akita, Hidetoshi Gon, Takumi Fukumoto, Akira Kikuchi
DOI:https://doi.org/10.1111/cas.15743
Supported by:
Grants-in-Aid for Scientific Research (S) and the AMED Next-Generation Cancer Creation Strategy.